Abstract
Introduction: Rituximab (R) plus CHOP (R-CHOP) is standard of care for patients (pts) with previously untreated DLBCL. Although most pts have long-term responses, up to 40% of pts fail to achieve a remission or relapse. Atezolizumab (atezo) is a fully humanized anti-programmed death-ligand 1 (PD-L1) antibody with a complementary mechanism of action to R. An ongoing Phase I/II study (NCT02596971) is evaluating the safety and efficacy of atezo in combination with R-CHOP (R-CHOP-atezo) in DLBCL pts. Results from the primary analysis are reported.
Methods: This open-label, multicenter study enrolled pts (≥18 years; ECOG PS 0-2) with previously untreated advanced DLBCL (Ann Arbor Stage III/IV, International Prognostic Index [IPI] score ≥2 or Stage II with bulky disease [at least 1 lesion ≥7cm]). Pts received induction treatment with R-CHOP-atezo (8 cycles [each 21 days] of R [375mg/m2 IV on Day 1 (Cycles 1-8)] and atezo [1200mg IV on Day 1 (Cycles 2−8)], and 6 or 8 cycles of CHOP [as determined by the investigator (INV)]). Pts who achieved a complete response (CR) at end of induction (EOI) received consolidation treatment with atezo 1200mg IV on Day 1 of Cycles 9─25, every 21 days for 12 months (mo). Primary endpoints were safety, and efficacy as determined by CR rate at EOI by independent review committee (IRC) using modified Lugano 2014 criteria (if bone marrow [BM] involvement at baseline [BL], CR was confirmed with a negative BM biopsy at EOI; designation of partial response [PR] on PET required that CR or PR on CT scan were met). Secondary endpoints included CR at EOI assessed by INV (modified Lugano 2014 criteria) and by IRC and INV (Cheson et al. J Clin Oncol 2007). Minimal residual disease (MRD) was evaluated at EOI using the Adaptive ImmunoSEQ® NGS platform (v2).
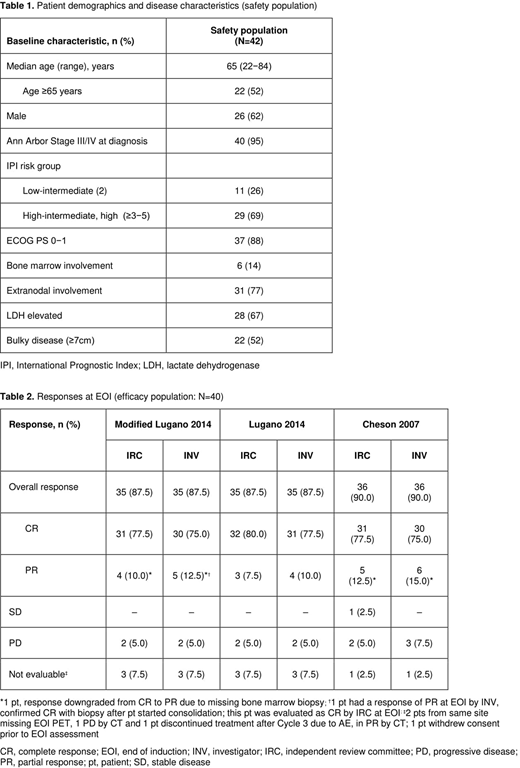
Results: At the data cut-off (April 11, 2018), 42 pts were enrolled and received treatment (safety population); 7 pts discontinued during the induction phase (1 protocol violation, 1 withdrawal of consent [these 2 pts discontinued after Cycle 1, Day 1 and were not treated with atezo, and were not included in the efficacy analysis], 4 adverse events [AEs], 1 progressive disease [PD]). Of 35 pts completing induction, 30 entered the consolidation phase; 5 pts discontinued at EOI (2 PD, 3 PR). Pt demographics and disease characteristics are shown in Table 1. Among 40 pts evaluable for response (efficacy population), 31 pts (77.5%) had a CR and 4 pts (10%) had a PR by IRC (modified Lugano 2014 criteria); PD occurred in 2 pts (5%), and 3 pts (7.5%) were not evaluable. Response assessments by Cheson et al. (J Clin Oncol 2007) were similar by IRC and INV; IRC assessment showed 31 (77.5%) CRs, 5 (12.5%) PRs, 2 (5%) pts with PD and 1 with SD (Table 2). MRD was evaluable in 26 pts at BL: 10 pts were MRD negative and 16 pts were MRD positive. Of the 16 MRD positive pts at BL, MRD data were available for 14 at EOI: 13 pts were negative and 1 pt was positive (PR by INV and IRC). Median dose intensity was >99% for the induction phase (median exposure 6.7 mo [range 1.5-7.3]) and 100% for the consolidation phase (median exposure 3.7 mo [range 0.7-9.3]). During induction, all 42 pts (100%) had ≥1 AE, 29 (69%) had a grade (Gr) 3-4 AE and 12 (29%) had a serious AE. No fatal AEs were reported. AEs led to any treatment discontinuation in 6 pts (14%) (Gr 3 neutropenia and Gr 3 transaminase increase, Gr 2 hyperthyroidism, Gr 3 lipase increase, Gr 1-2 peripheral neuropathy, Gr 4 thrombocytopenia), dose reduction (CHOP) in 9 pts (21%) (Gr 4 anemia, Gr 3 pancytopenia, Gr 2 paresthesia, Gr 4 neutropenia and Gr 1-2 peripheral neuropathy) and dose interruption (missed doses and dose delays) in 15 pts (36%). Five pts had AEs of interest to atezo (Gr 3 increased lipase, Gr 2 hyperthyroidism, and Gr 1 infusion-related reaction). Common Gr 3-4 AEs during induction were: neutropenia (45%), febrile neutropenia (9.5%), leukopenia (5%), anemia (5%), increased lipase (5%), and fatigue (5%), and during consolidation were: neutropenia (10%), febrile neutropenia (3%), increased lipase (14%), and syncope (7%).
Conclusions: The PET-CR rate with R-CHOP-atezo at EOI is encouraging and compares favorably with that previously reported with R-CHOP. The overall safety profile of R-CHOP-atezo is manageable, with no new safety signals reported. Preliminary MRD data at EOI are encouraging and support activity. Biomarker data and duration of response will be presented.
Younes:Sanofi: Honoraria; Roche: Honoraria, Research Funding; Takeda: Honoraria; Bayer: Honoraria; J&J: Research Funding; BMS: Honoraria, Research Funding; Astra Zeneca: Research Funding; Celgene: Honoraria; Novartis: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Honoraria; Janssen: Honoraria, Research Funding; Abbvie: Honoraria; Genentech: Research Funding; Incyte: Honoraria; Merck: Honoraria; Curis: Research Funding. Burke:Tempus Labs: Consultancy; Celgene: Consultancy; Gilead: Consultancy; Genentech: Consultancy; Abbvie: Consultancy; Bayer: Consultancy; Seattle Genetics: Consultancy, Speakers Bureau. Cheson:AbbVie, Roche/Genentech, Pharmacyclics, Acerta, TG Therapeutics: Consultancy. Diefenbach:Genentech: Consultancy; Trillium: Research Funding; Millenium/Takeda: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Incyte: Research Funding; Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Denovo: Research Funding; Acerta: Research Funding. Hawkes:Celgene: Other: Advisory board, Research Funding; Astra Zeneca: Research Funding; Merck: Other: Advisory board; Takeda: Other: Speaker fee; Roche: Other: Speaker fee; advisory board; Bristol Myers Squibb: Other: Speaker fee, Research Funding; Merck Sharpe Dohme: Research Funding; Merck KGA: Research Funding. Khan:Roche: Honoraria; AbbVie: Honoraria. Lossos:Affimed: Research Funding. Vitolo:Takeda: Speakers Bureau; Gilead: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sandoz: Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Yuen:Seattle Genetics: Research Funding. Oestergaard:Roche: Employment, Other: Ownership interests PLC. Chitra:Genentech/Roche: Employment. Wenger:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership, Other: Ownership interests PLC. Nielsen:F. Hoffmann-La Roche Ltd: Employment, Other: Ownership interests PLC. Sellam:Roche: Employment. Sharman:Pharmacyclics, an AbbVie Company: Consultancy, Research Funding; Acerta: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal